Since vaccines are given to previously healthy hosts, who may have never developed [autoimmune] disease had they not been immunized, adverse events should be carefully accessed and evaluated even if they represent a limited number of occurrences.
In this review of the literature, there is evidence of vaccine-induced autoimmunity and adjuvant-induced autoimmunity in both experimental models as well as human patients.
Scientific findings suggest that autoimmunity may be triggered by vaccine adjuvants, of which aluminum compounds (aluminum hydroxide and phosphate) have been the most studied and the most widely used. Adjuvants are molecules, which, in combination with antigens, enhance immunological response.
Adjuvants approved to date for human vaccines are: aluminum, MF59 in some viral vaccines, MPL, AS04, AS01B and AS02A against viral and parasitic infections, virosomes for hepatitis B virus (HBV), human papilloma virus (HPV), hepatitis A virus (HAV), and cholera toxin for cholera.
Aluminum nanoparticles have both a unique capacity of surpassing the blood brain barrier (BBB) and of eliciting immune inflammatory responses. These are probably the reasons why Aluminums’ most sensitive target is the brain, and also why documented side effects are mostly neurologic or neuropsychiatric.
It has been described as a neurotoxin because even when a relatively small amount of aluminium reaches the brain, is can act as a genotoxin, a prooxidant, it can be proinflammatory, act as an immunotoxin and also as an endocrine disruptor.
Aluminum interferes with many essential cellular processes. Memory, concentration, speech deficits, impaired psychomotor control, reduced seizure tolerance and altered behaviour are manifestations of aluminium neurotoxicity.
Moreover, Alzheimer’s, amyotrophic lateral sclerosis, Parkinsonism dementia, multiple sclerosis, and neurological impairments in children have been linked to aluminum neurotoxicity.
The vast majority of people are consuming higher amounts of aluminum through dietary and parenteral intake than what expert authorities consider safe.
Upper limits set by US food and drug administrations (FDA) for aluminum in vaccines are set at no more than 850 mcg/dose. These values were not based on toxicity studies, but on the minimum amount needed for aluminum to exert its effect as an adjuvant.
The quantities of aluminum to which infants, in their first year of age are exposed, have been considered safe by the FDA. However the scientific basis for this recommendation does not take into account aluminum persistence in the body.
The amount of dietary intake of aluminum has risen in urban societies to up to 100 mg/day considering the widespread use of processed convenience foods. However, only about 0.25% of dietary aluminum is absorbed into systemic circulation and most of it is thereafter eliminated through the kidneys. Absorption of aluminum by the skin from ointments and cosmetics containing aluminum has been shown. Moreover, the presence of aluminum in breast tissue was associated with breast cancer.
Aluminum compounds persist for up to 8–11 years post vaccination in human body. This fact, combined with repeated exposure, may account for a hyper activation of the immune system and subsequent chronic inflammation.
The clinical and experimental evidence collected so far identify at least three main risks associated with aluminum in vaccines:
1. It can persist in the body.
2. It can trigger pathological immunological responses.
3. It can pass through the BBB into the CNS where it can trigger immuno-inflammatory processes, resulting in brain inflammation and long-term neural dysfunction.
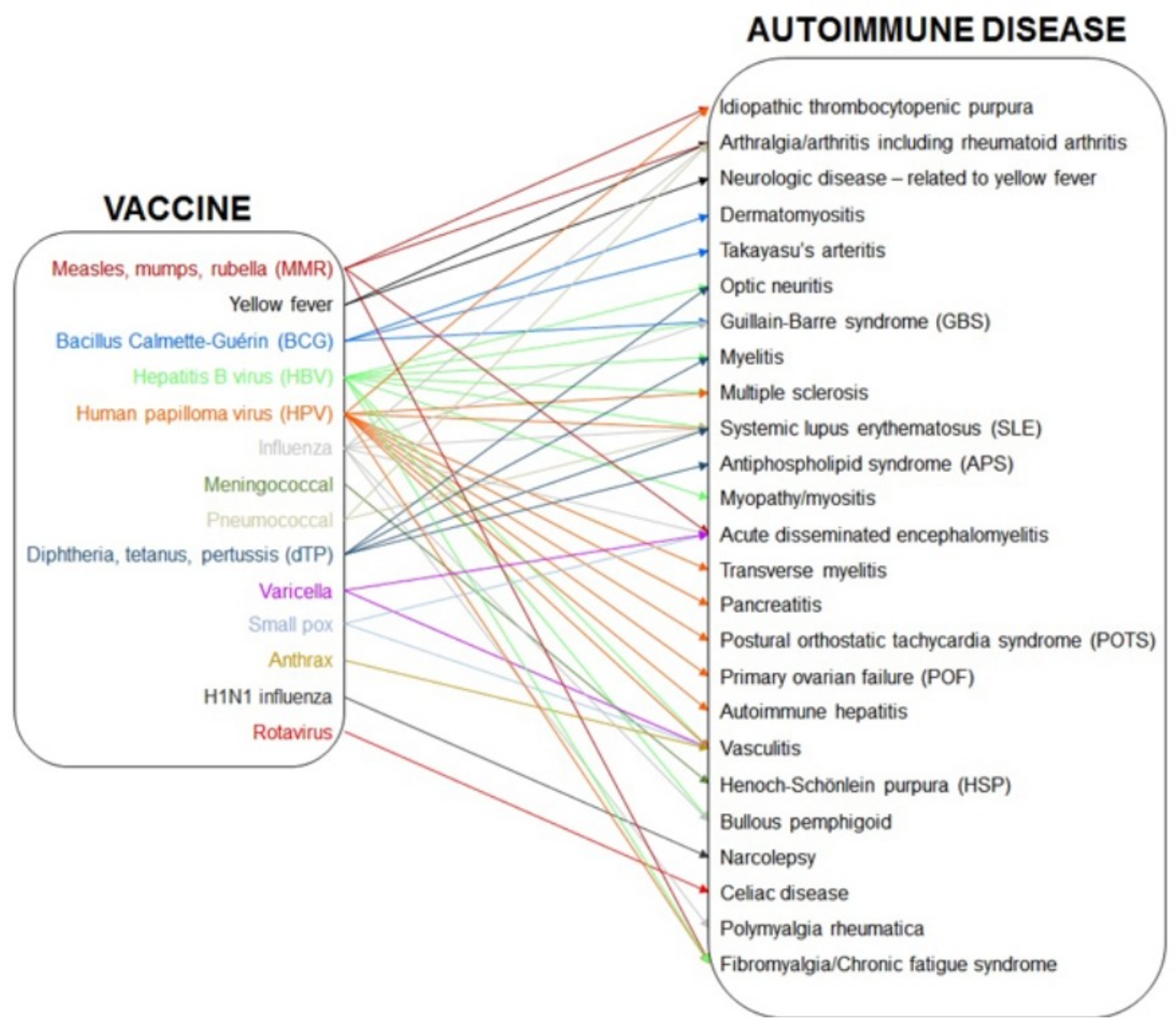
The [Hepatitis B / HBV] vaccine has been associated mainly with autoimmune neuromuscular disorders. They include, but are not limited to: optic neuritis, Guillain-Barre syndrome (GBS), myelitis and multiple sclerosis (MS), systemic lupus erythematosus (SLE), arthritis, vasculitis, antiphospholipid syndrome (APS) and myopathy. HBV vaccine is the most common immunization associated with acute myelitis.
HPV poses a special challenge in vaccine safety. [M]ost women infected with HPV will not develop the disease since 70% of infections will resolve within a year and up to 90% within 2 years without specific treatment. Over the course of decades, cancer may result in a small proportion of the remaining infected women. Death rate from cervical cancer in 9–20 year old girls is zero and long-term benefits are yet to be proven.
There have been several reports of post-licensure adverse events, some of which have even been fatal. Compared to other vaccines, an unusually high proportion of adverse drug reactions has been reported associated with HPV vaccines.
[S]everal autoimmune diseases have been linked to HPV immunization. Examples include GBS, MS, Acute disseminated encephalomyelitis (ADEM), Transverse Myelitis (TM), postural orthostatic tachycardia syndrome (POTS), SLE, primary ovarian failure (POF), pancreatitis, vasculitis, immune thrombocytopenic purpura (ITP) and Autoimmune hepatitis (AH).
Diseases previously associated with meningococcal vaccines are GBS, Henoch-Schönlein Purpura (HSP) and Bullous pemphigoid (BP) .
Vaccine adverse events [for pneumococcal vaccines] vary depending on whether the vaccine is adjuvanted or not. In a non adjuvanted vaccine, local reactions are present in 9% of people vaccinated intra muscularly and in 24% of those immunized subcutaneously. In conjugated vaccines, this percentage rises to 50%. Systemic reactions such as fever, irritability, decreased appetite and sleep disturbances occur in 80–85% of recipients of PCV or PPV. Symptoms like arthralgia, arthritis, myalgia, paresthesia and fatigue are more frequent in patients post PPV.
As many as 1% of recipients of aluminum containing adjuvants may be sensitized to future exposure.
ASIA syndrome and aluminum safety studies show that the use of aluminum containing “placebo” in control groups in vaccine safety studies should be carefully evaluated. New studies must be performed using a proper placebo to adequately test vaccine safety. Another evident failure in vaccine safety studies are the short-term periods which are evaluated.